
2021 has already passed, a year when Allist forged ahead against all odds and reaped great success. As we look back on 2021, we can see from the following events that every decisive footstep taken by Allist was a move in the right direction in terms of clinical development and commercialization.
1. BREAKTHROUGH! Positive results from registry study for the first-line treatment of non-small cell lung cancer (NSCLC) with Furmonertinib
l On November 1, the clinical study on the first-line treatment with Furmonertinib for advanced non-small cell lung cancer (NSCLC) (FURLONG) achieved a primary study endpoint of progression-free survival (PFS), and the Furmonertinib-treated arm resulted in a statistically significant and clinically significant PFS benefit compared to the control arm.
l On December 1, Furmonertinib was granted a breakthrough therapy designation by the NMPA Center for Drug Evaluation (CDE) for the first-line treatment of advanced non-small cell lung cancer (NSCLC).
l On December 8, Furmonertinib was included in the public list of NMPA priority review drugs for the first-line treatment for advanced non-small cell lung cancer (NSCLC).
l On December 13, the marketing application of Furmonertinib for the first-line treatment of advanced non-small cell lung cancer (NSCLC) was officially accepted by the CDE.
2. GOOD NEWS! Furmonertinib is added to China's national medical insurance catalog
On December 3, 2021, the National Healthcare Security Administration and the Ministry of Human Resources and Social Security released the "National Medicine List for Basic Medical Insurance, Work-related Injury Insurance and Maternity Insurance (2021)", for which Furmonertinib was successfully included in the 2021 version of the Catalog of Medicines Covered by the National Medical Insurance System following the current round of medical insurance negotiations. This is a major milestone in the commercialization of Furmonertinib, which will bring benefits to more patients with advanced lung cancer in China by enhancing the accessibility and affordability of Furmonertinib.
3. GOING INTERNATIONAL! The overseas authorization of Furmonertinib is completed and it is heading abroad

On June 30, Allist and ArriVent Biopharma, Inc. in the U.S. reached an exclusive overseas licensing agreement for Furmonertinib, whereby Allist will receive a $40 million down payment and will be eligible for potential registration and sales milestone payments, as well as a commission on overseas sales.
4. COOPERATION! University-enterprise collaboration to develop the first-in-class drug

On July 28, Allist signed a technology transfer agreement with Fudan University to obtain all the study results of the KH3 project for Phosphoglycerate Mutase 1 (PGAM1) Inhibitor developed by the School of Pharmacy of Fudan University, as well as the corresponding domestic and foreign patent rights and all the rights and interests of domestic and foreign development, registration and sales. Allist's product pipeline will be strategically expanded from lung cancer to other oncology diseases.
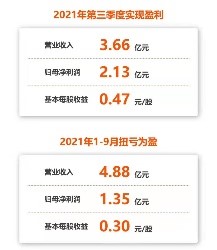
5. PROFIT! Allist generates profits in 2021
6. PRODUCTION EXPANSION! The production capacity of Jiangsu Allist has been increased
Jiangsu Allist is an innovative drug industrialization base invested in by Shanghai Allist Pharmaceutical Technology Co., Ltd. As market sales saw a continuous increase, the batch size was changed to 200,000 tablets per batch to cater to the market supply. The process validation production of three consecutive batches of 200,000 tablets per batch was conducted in October 2021. All materials used in the production process and the intermediate products from each process step all met the defined standards. The test results of the finished products met the defined specifications, which confirmed the consistency of product quality before and after the batch size change. This batch size change doubled the previous production capacity and reduced the production costs further.

7. INITIATION! Furmonertinib enters the field of early lung cancer treatment
l On January 11, the clinical trial on Furmonertinib for post-operative adjuvant therapy in EGFR-positive non-small cell lung cancer (NSCLC) patients was approved, marking official entry of Allist into the field of early lung cancer treatment.
l In April, the clinical study on Furmonertinib for post-operative adjuvant therapy in EGFR-positive non-small cell lung cancer (NSCLC) patients (FORWARD) was initiated, allowing Allist to advance at full speed the field of early lung cancer treatment.

8. MAKING AN APPEARANCE! Impressive data was viewed on the international stage
l Study data on Furmonertinib for the treatment of brain metastases in non-small cell lung cancer (NSCLC) was presented at 2020 WCLC on January 13.
l In March, the Phase IIb clinical study on Furmonertinib was published in the top international medical journal, "The Lancet Respiratory Medicine", becoming the first original third-generation EGFR-TKI in China to have its Phase IIb pivotal registration clinical study successfully published.
l In September, data on Furmonertinib for EGFR exon 20 insertion mutations was published for the first time by the European Society for Medical Oncology (ESMO).

9. FORTUNE! Rapid commercialization of Furmonertinib benefits more patients
In March 2021, Furmonertinib was commercially marketed in China, becoming the world's third and China's second targeted innovative drug approved for the treatment of EGFR-T790M mutated non-small cell lung cancer (NSCLC). At present, Furmonertinib has covered 30+ provinces and cities, 1000+ hospitals and about 300+ DTP pharmacies nationwide.
Furmonertinib has also been incorporated in inclusive commercial insurance to benefit more Chinese patients. Currently, it has been successfully introduced in inclusive commercial medical insurance in 47 cities, so that more Chinese patients with non-small cell lung cancer (NSCLC) can benefit from Allist's Furmonertinib.
10. HONORS! Allist has won several awards
l On April 7, 2021, Shanghai Allist Pharmaceutical Technology Co., Ltd. was ranked in the TOP 10 of the best listed biopharmaceutical companies in 2020.
l On June 10, 2021, Shanghai Allist Pharmaceutical Technology Co., Ltd. and Mr. Du Jinhao, Chairman of the Board of Directors, were awarded "TOP 10 New Drug Innovation Companies of the Year" and "TOP 10 Drug Innovation Leaders of the Year", respectively.
l On August 1, 2021, the 38th China Pharmaceutical Industry Information Annual Conference 2021 hosted by the China National Pharmaceutical Industry Information Center kicked off in Jinan City, Shandong Province. Boasting outstanding innovative R&D capabilities, Allist was awarded the title of one of "China's New Innovative Forces in Pharmaceuticals by 2021".
l On December 21, 2021, Allist was awarded the title of "TOP 100 China Pharmaceutical Innovation Enterprises 2021" at the prestigious China Healthcare Summit of Entrepreneurs, Scientists and Investors. Committed to all-round innovation and offering unique advantages and sustainability in the field of pharmaceutical innovation, Allist was ranked on the list of the Top 100 innovative enterprises for two consecutive years.

